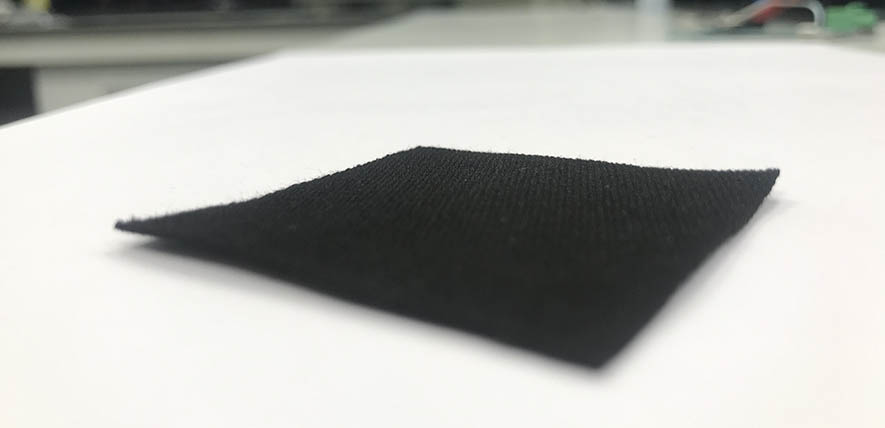
Researchers from the University of Cambridge used a method similar to charging a battery to instead charge activated charcoal, which is often used in household water filters.
By charging the charcoal ‘sponge’ with ions that form reversible bonds with CO2, the researchers found the charged material could successfully capture CO2 directly from the air.
The charged charcoal sponge is also potentially more energy efficient than current carbon capture approaches, since it requires much lower temperatures to remove the captured CO2 so it can be stored. The results are reported in the journal Nature.
“Capturing carbon emissions from the atmosphere is a last resort, but given the scale of the climate emergency, it’s something we need to investigate,” said Dr Alexander Forse from the Yusuf Hamied Department of Chemistry, who led the research. “The first and most urgent thing we’ve got to do is reduce carbon emissions worldwide, but greenhouse gas removal is also thought to be necessary to achieve net zero emissions and limit the worst effects of climate change. Realistically, we’ve got to do everything we can.”
Direct air capture, which uses sponge-like materials to remove carbon dioxide from the atmosphere, is one potential approach for carbon capture, but current approaches are expensive, require high temperatures and the use of natural gas, and lack stability.
“Some promising work has been done on using porous materials for carbon capture from the atmosphere,” said Forse. “We wanted to see if activated charcoal might be an option, since it’s cheap, stable and made at scale.”
Activated charcoal is used in many purification applications, such as water filters, but normally it can’t capture and hold CO2 from the air. Forse and his colleagues proposed that if activated charcoal could be charged, like a battery, it could be a suitable material for carbon capture.
When charging a battery, charged ions are inserted into one of the battery’s electrodes. The researchers hypothesised that charging activated charcoal with chemical compounds called hydroxides would make it suitable for carbon capture, since hydroxides form reversible bonds with CO2.
The team used a battery-like charging process to charge an inexpensive activated charcoal cloth with hydroxide ions. In this process, the cloth essentially acts like an electrode in a battery, and hydroxide ions accumulate in the tiny pores of the charcoal. At the end of the charging process, the charcoal is removed from the “battery”, washed and dried.
Tests of the charged charcoal sponge showed that it could successfully capture CO2 directly from the air, thanks to the bonding mechanism of the hydroxides.
“It’s a new way to make materials, using a battery-like process,” said Forse. “And the rates of CO2 capture are already comparable to incumbent materials. But what’s even more promising is this method could be far less energy-intensive, since we don’t require high temperatures to collect the CO2 and regenerate the charcoal sponge.”
To collect the CO2 from the charcoal so it can be purified and stored, the material is heated to reverse the hydroxide-CO2 bonds. In most materials currently used for CO2 capture from air, the materials need to be heated to temperatures as high as 900°C, often using natural gas. However, the charged charcoal sponges developed by the Cambridge team only require heating to 90-100°C, temperatures that can be achieved using renewable electricity. The materials are heated through resistive heating, which essentially heats them from the inside out, making the process faster and less energy-intensive.
The materials do, however, have limitations that the researchers are now working on. “We are working now to increase the quantity of carbon dioxide that can be captured, and in particular under humid conditions where our performance decreases,” said Forse.
The researchers say their approach could be useful in fields beyond carbon capture, since the pores in the charcoal and the ions inserted into them can be fine-tuned to capture a range of molecules.
“This approach was a kind of crazy idea we came up with during the Covid-19 lockdowns, so it’s always exciting when these ideas actually work,” said Forse. “This approach opens a door to making all kinds of materials for different applications, in a way that’s simple and energy-efficient.”
A patent has been filed and the research is being commercialised with the support of Cambridge Enterprise, the University’s commercialisation arm.
The research was supported in part by the Leverhulme Trust, the Royal Society, the Engineering and Physical Sciences Research Council (EPSRC), part of UK Research and Innovation (UKRI), and the Centre for Climate Repair at Cambridge.
Reference:
Huaiguang Li et al. ‘Capturing carbon dioxide from air with charged sorbents.’ Nature (2024). DOI: 10.1038/s41586-024-07449-2
“The University of Cambridge is a public collegiate research university in Cambridge, England. Founded in 1209, the University of Cambridge is the third-oldest university in continuous operation.”
Please visit the firm link to site